HRT assessment of risks and benefits based on age
Last edited 02/2020 and last reviewed 07/2022
clinical indications for HRT
Current guidelines recommend HRT for perimenopausal and early postmenopausal women without contraindications, who are experiencing troublesome vasomotor symptoms.
Age of menopause is an important factor when considering HRT. Generally for women with menopausal symptom who are
- <50 years - HRT should be offered since the benefits far outweigh the risks
- between 50 and 60 years - the benefits of HRT outweigh the risks
- >60 years - benefits of HRT equal the risks and treatment should be individualised
- >70 years - risks tend to outweigh the benefits (1)
Although HRT decreases the fracture risk and may also improve mood and libido, these are not considered as primary indications for treatment (1).
For women with vaginal symptoms alone, a local oestrogen is adequate to manage the symptoms (1).
Notes:
- detailed information about risks of HRT for 50-59 year old women or less than 10 years after menopause (2)
- primary areas of benefit included relief of hot flashes and symptoms of urogenital atrophy and prevention of fractures and diabetes
- risks included venothrombotic episodes, stroke, and cholecystitis
- in the subgroup of women starting Menopausal Hormone Therapy between ages 50 and 59 or less than 10 yr after onset of menopause, congruent trends suggested additional benefit including reduction of overall mortality and coronary artery disease
- in this subgroup, estrogen plus some progestogens increased the risk of breast cancer, whereas estrogen alone did not
- beneficial effects on colorectal and endometrial cancer and harmful effects on ovarian cancer occurred in the estrogen plus progestogen group but affected only a small number of women
- beneficial effects on colorectal and endometrial cancer and harmful effects on ovarian cancer occurred in the estrogen plus progestogen group but affected only a small number of women
- in this subgroup, estrogen plus some progestogens increased the risk of breast cancer, whereas estrogen alone did not
Benefits and Risks of Menopausal Hormonal Therapy (MHT) in Women Recently Menopausal (i.e. ages 50-59 or <10 yr postmenopausal)
- reanalyses of the Women's Health Initiative (WHI) indicated the important influences of age and time since initiation of MHT on benefits and risks. Because most women start MHT shortly after menopause, available data regarding these women were specifically analyzed. Results are summarized as the excess number of women experiencing benefit or risk per 1000 women using MHT for 5 yr of more. Because no randomized controlled trials were available to determine these estimates, conclusions are tentative
- Benefits of Estrogen Alone (excess number of women per 1000 per 5 yr of use who experienced event attributable to use of MHT)
- Excess number
- 0-1None
- 1.1-5 Reduction in breast cancer, coronary heart disease
- 5.1-10 Reduction in fractures, overall mortality
- >10 Reduction in type 2 diabetes
- Excess number
- Benefits of Estrogen Plus a Progestogen
- Excess number
- 0-1 Reduction in coronary heart disease (subgroup <10 yr postmenopausal), endometrial cancer
- 1.1-5 Reduction in fractures, colorectal cancer
- 5.1-10 Reduction in overall mortality
- >10 Reduction in type 2 diabetes
- Harm from Standard Oral Estrogen Alone
- Excess number
- 0-1 Increase in colorectal cancer , ovarian cancer
- 1.1-5 Increase in venothrombotic episodes, stroke
- 5.1-10 None
- >10 Increase in cholecystitis
- Excess number
- Harm from Oral Estrogen Plus a Progestogen
- Excess number
- 0-1 Increase in stroke
- 1.1-5 Increase in coronary heart disease (subgroup ages, 50-59 yr)
- 5.1-10 Increase in breast cancer, venothrombotic episodes, cholecystitis
- >10 None
- Excess number
- Benefits of Estrogen Alone (excess number of women per 1000 per 5 yr of use who experienced event attributable to use of MHT)
- primary areas of benefit included relief of hot flashes and symptoms of urogenital atrophy and prevention of fractures and diabetes
Breast Cancer and HRT Update (3,4):
A study has showed that an increased risk of breast cancer with HRT is similar whether HRT is taken orally (swallowed) or delivered via patches or gels or implants (3)
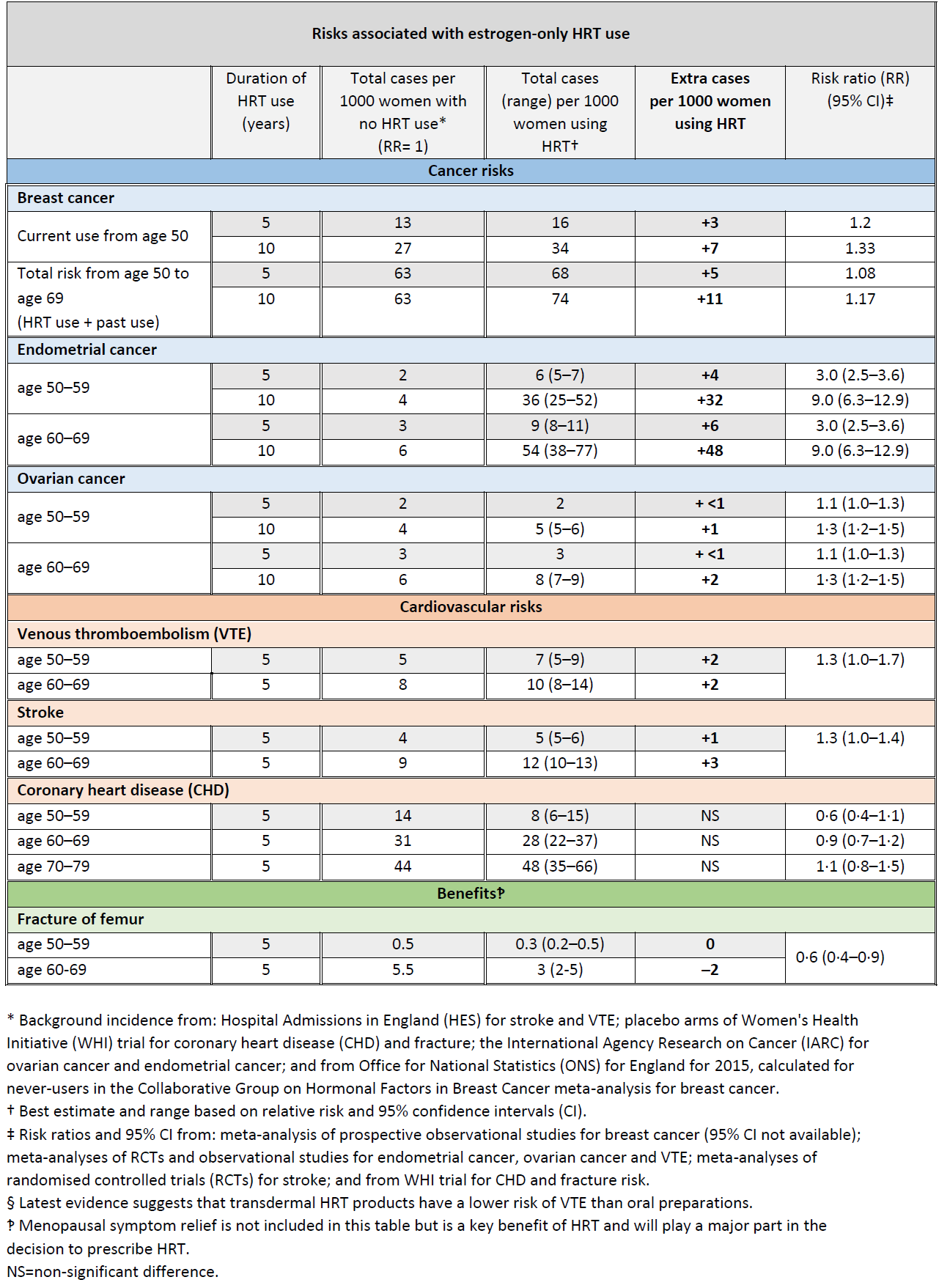
In the UK about 1 in 16 women who never use HRT are diagnosed with breast cancer between the ages of 50 and 69 years.
This is equal to 63 cases of breast cancer per 1000 women. Over the same period (ages 50-69 years), with 5 years of HRT use, the study estimated:
- about 5 extra cases of breast cancer per 1000 women using estrogen-only HRT
- about 14 extra cases of breast cancer per 1000 women using estrogen combined with progestogen for part of each month (sequential HRT)
- about 20 extra cases of breast cancer per 1000 women using estrogen combined with daily progestogen HRT (continuous HRT) These risks are for 5 years of HRT use.
The numbers of extra cases of breast cancer above would approximately double if HRT was used for 10 years instead of 5.
There was no increased risk of breast cancer associated with use of vaginal oestrogen preparations (3)
The MHRA have produced updated guidance relating to the risks and benefits of HRT (4):

Reference:
- (1) Hickey M, Elliott J, Davison SL.Hormone replacement therapy. BMJ. 2012;344:e763.
- (2) Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab2010;95(7 suppl 1):s1-66
- (3) Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. The Lancet. Published August 29, 2019.
- (4) MHRA (August 2019). Hormone replacement therapy and risk of breast cancer