UKONS
Last edited 04/2020 and last reviewed 05/2023
BACKGROUND
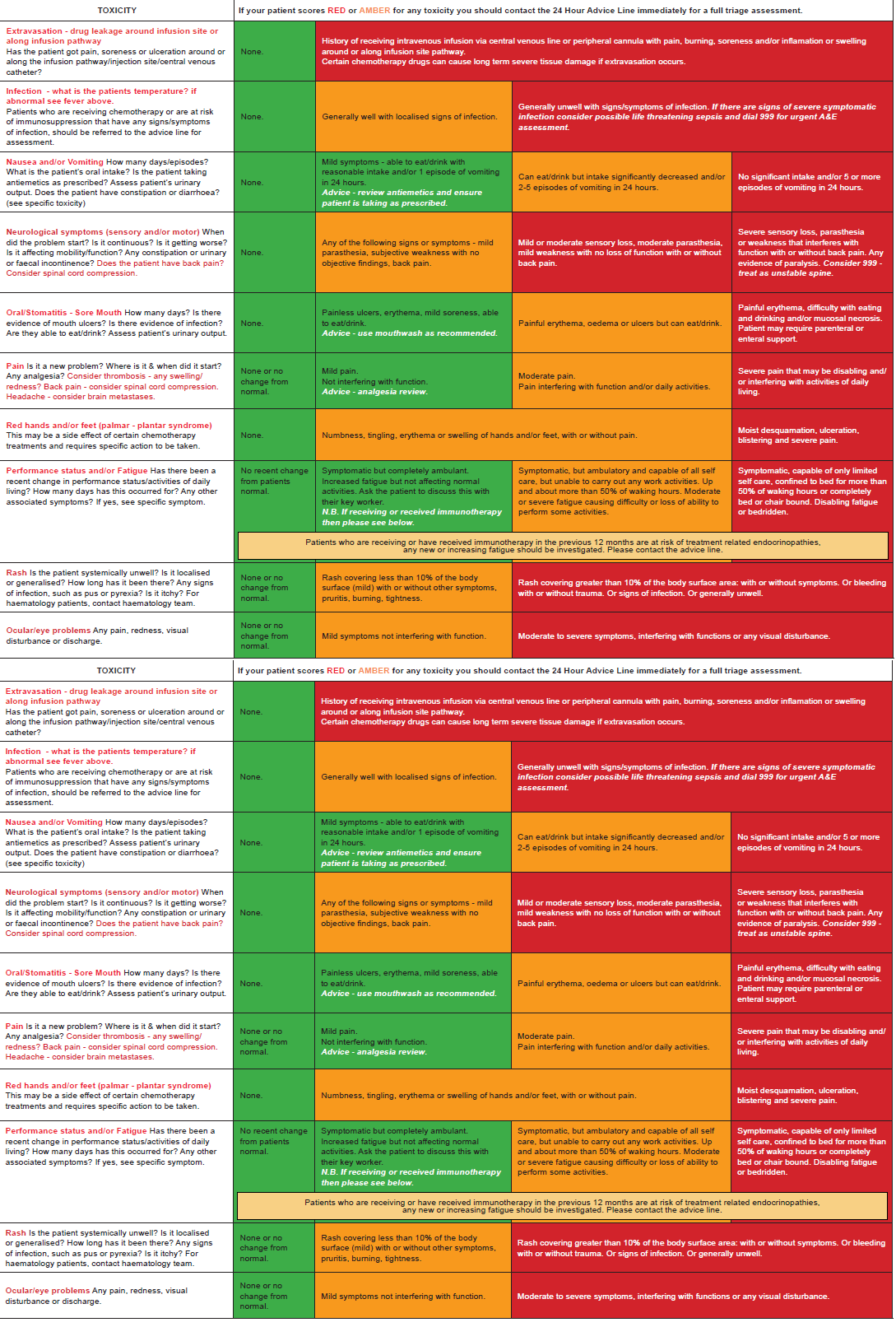
Primary Care Risk Assessment Tool for Oncology/Haematology Patients who are:
- Receiving or received systemic anti-cancer therapies
- Receiving or recently received radiotherapy.
- At risk of disease related immunosuppression.
It is important that the side effects of treatment are not underestimated and that the significance of symptoms is recognised.
This evidence-based risk assessment tool grades the presenting symptoms and advises action accordingly using a RAG system. It is important that the significance of lower level amber toxicities are recognised.
Systemic anti cancer therapy is an overarching term that includes cytotoxic chemotherapy, immunotherapy, monoclonal antibodies and new novel therapies.
RISK ASSESSMENT PROCESS
- all patients receiving Systemic Anti-Cancer Therapy are provided with a 24 hour advice line telephone number
- this tool is used to risk assess any symptom the patient mentions to you. Patients might only report symptoms that are most worrying to them, and not mention others that may be significant. It is very helpful to use the risk assessment as a quick checklist to identify any potential problems
- If the patient scores RED or AMBER for any symptom, the primary care clincian should contact the 24 Hour Advice Line immediately for a full triage assessment, unless URGENT referral to A&E is advised
- patients may require urgent assessment in a suitable clinical area that provides access to investigation and treatment facilities. The advice line team will arrange assessment and/or further monitoring for the patient, if they feel it is required
- please be aware that the period of time that patients may experience post treatment side effects/complications may vary according to the treatment they have received, and can be as late as 12 months post treatment
- patients may present with problems other than those listed. Be cautious, and if in doubt about anything contact the advice line.
Reference: