management
Last edited 08/2021 and last reviewed 07/2022
Therapy of GORD can be divided into: (1)
Dietary measures:
- thickened feedings – useful in reducing regurgitation and vomiting in infants and children under 2 years, rice, or corn can be used as feed thickeners (2)
- frequent small meals – for older infants and children (2)
- removal of cow’s milk protein from diet – in infants with cow’s milk protein allergy (3)
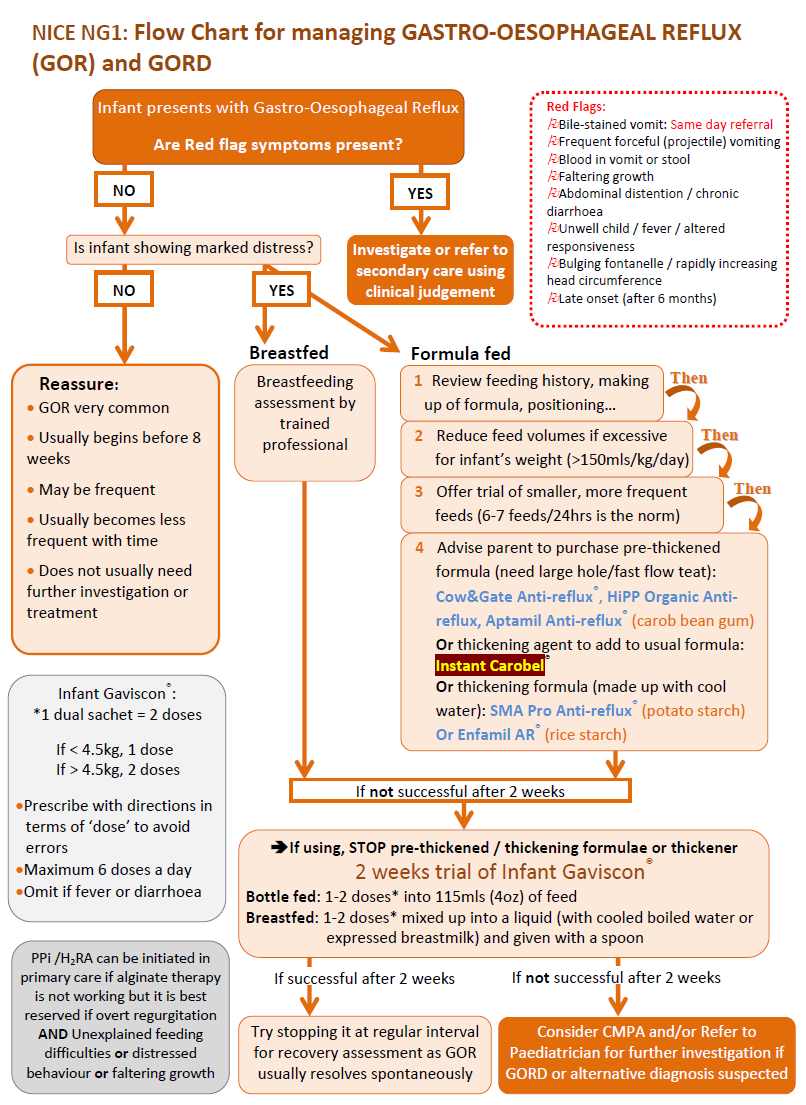
- NICE outline a stepped-care approach (8):
- in formula-fed infants with frequent regurgitation associated with marked
distress, use the following stepped-care approach:
- review the feeding history, then
- reduce the feed volumes only if excessive for the infant's weight, then
- offer a trial of smaller, more frequent feeds (while maintaining an appropriate total daily amount of milk) unless the feeds are already small and frequent, then
- offer a trial of thickened formula (for example, containing rice starch, cornstarch, locust bean gum or carob bean gum)
- in formula-fed infants with frequent regurgitation associated with marked
distress, use the following stepped-care approach:
Positioning:
- GOR is less when placed in the prone position (when infant is awake, specially during post prandial period) compared to supine position (due to the increased risk of sudden infant death syndrome supine position is recommended during sleep) (3)
- seated positioning may increase intra-abdominal pressure and cause reflux and should be avoided (2)
- for children older than 1 year left side positioning and elevation of the head of the bed may be beneficial (3)
- NICE state that do not use positional management to treat GOR in sleeping infants. In line with NHS advice, infants should be placed on their back when sleeping (8)
Pharmacological:
- antacids (e.g. gaviscon) may be indicated
- in formula-fed infants, if the stepped-care approach is unsuccessful (see above), stop the thickened formula and offer alginate therapy for a trial period of 1-2 weeks. If the alginate therapy is successful continue with it, but try stopping it at intervals to see if the infant has recovered
- in breast-fed infants with frequent regurgitation associated with marked
distress that continues despite a breastfeeding assessment and advice,
consider alginate therapy for a trial period of 1-2 weeks. If the alginate
therapy is successful continue with it, but try stopping it at intervals
to see if the infant has recovered
- H2 antagonists e.g. randitidine are widely used in the management of this
condition
- ranitidine has been used extensively in the management of GORD in infancy and has a good safety record (2). It is available as an oral solution
- omeprazole (5)
- omeprazole capsules, tablets and oral suspension may be used in neonates, but they are not licensed for this age group
- omeprazole capsules, tablets and oral suspension may be used in children as detailed below, although these situations are considered unlicensed:
- gastro-oesophageal reflux disease and acid-related dyspepsia in children aged under 1 year and under 10 kg for capsules and tablets, and in children aged under 1 month for oral suspension
- domperidone has been used for its anti-emetic effects although it is now ot licensed for use in children for gastro-oesophageal reflux disease (5)
- metoclopramide may have some benefit in comparison to placebo in the symptomatic treatment for GORD, but that must be weighed against possible side effects (4)
NICE state (8):
-
do not offer acid-suppressing drugs, such as proton pump inhibitors (PPIs) or H2 receptor antagonists (H2RAs), to treat overt regurgitation in infants and children occurring as an isolated symptom
- consider a 4-week trial of a PPI or H2RA for children and young people with persistent heartburn, retrosternal or epigastric pain
- assess the response to the 4-week trial of the PPI or H2RA, and consider
referral to a specialist for possible endoscopy if the symptoms:
- do not resolve or
- recur after stopping the treatment
- offer PPI or H2RA treatment to infants, children and young people with endoscopy-proven reflux oesophagitis, and consider repeat endoscopic examinations as necessary to guide subsequent treatment
- do not offer metoclopramide, domperidone or erythromycin to treat GOR or GORD without seeking specialist advice and taking into account their potential to cause adverse events
Infants with GORD complicated by failure to thrive should receive shared care between primary and secondary services.
Surgery may be indicated if there is failure to thrive, oesophageal ulceration and recurrent or persistent aspiration
- consider fundoplication in infants, children and young people with severe,
intractable GORD if (8):
- appropriate medical treatment has been unsuccessful or
- feeding regimens to manage GORD prove impractical, for example, in the case of long-term, continuous, thickened enteral tube feeding
A flowchart for the management of GORD has been suggested (9):

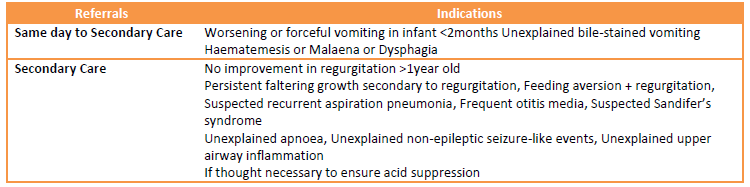
Onward referrals
Notes:
- conventional management has involved nursing the infant on a head-up sloping
board either in the prone or supine position. However a systematic review
provides evidence that elevating the head of the crib in the supine position
does not have any effect on GORD in children under two years of age (1)
- consider cow's milk protein intolerance (6)
- this is a cause of GORD in a proportion of babies not responding to first line treatments. Consider prescribing a 1-2 week trial of a hypoallergenic milk such as Nutramigen® (Give advice re milk-free solids if appropriate). Breast feeding mums can trial a milk- free diet under dietician supervision. If no obvious improvements in 1-2 weeks switch back to a normal milk. If improve and staying on a milk-free diet refer to the paediatric dieticians for ongoing advice. Most will be able to successfully reintroduce milk at the end of the first year. This should be done gradually starting with small amounts of cooked milk such as in 1/8 malted milk biscuit
- a negative skin prick test or specific IgE (RAST) does not rule out cows milk as the cause of the reflux.
- lactose intolerance tends to cause diarrhoea, abdominal pain and wind
rather than reflux in infants (so consider in 'silent refluxers' - 1-2
week trial of lactose free milk if bottle fed)
- use of ranitidine in infant GORD (6)
- if not improving with use of Gaviscon
- consider the use of Ranitidine, initially for a 4 week trial:
- ranitidine dosage: (Off-label prescribing of licensed product
under 3 years old see British National Formulary BNF for details).
Available as syrup 75mg/5ml
- under 6 months: 1-3mg/kg three times daily
- over 6 months: 2-4mg/kg twice daily. Max 150mg/dose.
- refer to current child BNF for full prescribing information.
It is best given at a separate time from Gaviscon
- ranitidine dosage: (Off-label prescribing of licensed product
under 3 years old see British National Formulary BNF for details).
Available as syrup 75mg/5ml
- consider the use of Ranitidine, initially for a 4 week trial:
- if not improving with use of Gaviscon
- changing from ranitidine to omeprazole (seek expert advice) (6)
- try this for 4-6 weeks, increasing to the highest dose if needed. only available as capsules/tablets - capsule contents can be mixed with fruit juice or yoghurt. Losec MUPS® can disperse in water. Tablets should not be crushed. If giving via a NGT/gastrostomy prescribe omeprazole capsules and dissolve in 10ml 8.4% sodium bicarbonate oral solution so that tube does not block.
- omeprazole
- dosage: (Off-label prescribing of licensed product, see BNF-C for
details)
- for neonate
- 700 micrograms/kg once daily for 7-14 days, then increased if necessary to 1.4-2.8mg/kg once daily
- for child 1 month-1 year
- 700 micrograms/kg once daily, increased if necessary to 3 mg/kg once daily (max. per dose 20 mg)
- omeprazole capsules, tablets and oral suspension may be used in children as detailed below, although these situations are considered unlicensed for gastro-oesophageal reflux disease and acid-related dyspepsia in children aged under 1 year and under 10 kg for capsules and tablets, and in children aged under 1 month for oral suspension (5)
- for neonate
- dosage: (Off-label prescribing of licensed product, see BNF-C for
details)
Notes:
- ranitidine therapy in very low birth weight (VLBW) infants
- there is evidence that ranitidine is associated with an increased risk of infections, necrotising enterocolitis (NEC), and fatal outcome in VLBW infants (7)
Reference:
- (1) Pritchard DS, Baber N, Stephenson T. Should domperidone be used for the treatment of gastro-oesophageal reflux in children? Systematic review of randomized controlled trials in children aged 1 month to 11 years old. Br J Clin Pharmacol. 2005;59(6):725-9
- (2) Jung A.D. Gastroesophageal Reflux in Infants and Children. Am Fam Physician. 2001;64(11):1853-60
- (3) Vandenplas Y et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). Journal of Pediatric Gastroenterology and Nutrition 2009; 49:498-547
- (4) Craig WR et al. Metoclopramide, thickened feedings, and positioning for gastro-oesophageal reflux in children under two years. Cochrane Database Syst Rev 2004; (4):CD003502.
- (5) BNF for Children (accessed August 3rd 2021)
- (6) Royal United Hospital Bath (February 2012). Gastro-Oesophageal Reflux in Infants Management Guidelines.
- (7) Terrin G et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns.Pediatrics. 2012 Jan;129(1):e40-5.
- (8) NICE (January 2015). Gastro-oesophageal reflux disease: recognition, diagnosis and management in children and young people
- (9) Wessex Infant Feeding Guidelines and Appropriate Prescribing of Specialist Infant Formulae (Accessed 8/3/2020)
referral criteria from primary care - gastro-oesophageal reflux disease (GORD) in infancy